Results of the Pinpoint Project

The Pinpoint web app was created and evaluated by Klein Buendel in collaboration with HPC International through a grant funded by the National Institute on Minority Health and Health Disparities (MD010746; Dr. Valerie Myers, Principal Investigator). Designed for adolescents aged 13-17, the Pinpoint web app provides education on communication strategies and sickle cell disease (SCD) care management. Pinpoint includes a pain assessment tool, vocabulary game, body scanner reflection, and educational self-disclosure activity. It was developed as an interactive gaming web app for use on smartphones, tablets, and desktop computers. Learning Buddies were added to act as a guide for the teen in the app to explain each activity and feature with text and voiceover. There are two Learning Buddy characters to choose from, each of whom had their own fictional story about living with SCD. Learning Buddies are customizable, allowing teen participants to choose clothing, hair style, and skin tone upon registering for the app. For each activity the teens participate in, points can be earned to unlock additional customization options, including hats, jewelry, and more hairstyles and clothing options. A Self-Disclosure Stories section was also added to the web app. This consists of stories from real people, including children, young adults, and older adults living with SCD.
The fully developed app was used to interview clinicians to evaluate content and clinical meaningfulness (n=10). Additionally, adolescents (n=11), aged 13-17, with SCD participated in usability testing to evaluate the user interface, ease of use, and perceived barriers. Both clinicians and teens participating in the usability study were encouraged to go through the app on their own while sharing their screen and describing aloud their thoughts and impressions of the app. They were then guided to specific parts of the app (such as the vocabulary game, self-disclosure activity, Pain Assessment Tool, Body Scanner Reflection, and Learning Buddies) to review.
Overall, clinicians believed the app content was meaningful and engaging, would help their patients better identify SCD pain and would help them better treat their patients’ pain. Clinicians also said they would encourage SCD patients to use the app. Teen participants in the usability testing found the app to be easy to use and understand. They enjoyed the interactivity of the games, found the Learning Buddy to be interesting and relatable, and liked that they could share the information recorded on the pain assessment tool with caregivers and healthcare providers by text or email.
In a subsequent randomized, stepped-wedge trial, the app was tested with 13–17-year-olds with SCD and one of their parents to evaluate changes in knowledge acquisition for communicating about pain. Community-based recruitment strategies were used. This involved attending SCD conferences, creating relationships with community-based organizations (such as sickle cell associations, sickle cell camps, libraries, Boys and Girls clubs, YMCAs, and clinics), online recruitment (such as Facebook/Reddit ads and posting in SCD-specific groups on Facebook/Reddit), partnering with companies that specialize in recruiting for SCD research, and snowball recruitment. Through these efforts, 24 teen/parent dyads were successfully recruited and randomly assigned to study group.
Both teens and their parent took a survey every 4 weeks for 12-16 weeks and used the app for 4-12 weeks, depending on which arm of the study they were assigned. Parents and teens randomized into Arms 1-3 took surveys every 4 weeks for 12 weeks and downloaded the Pinpoint app at Baseline (Arm 1; used the app for 12 weeks), 4 Weeks (Arm 2; used the app for 8 weeks), or 8 Weeks (Arm 3; used the app for 4 weeks), while participants in Arm 4 took surveys every 4 weeks for 16 weeks and downloaded the Pinpoint app at 12 Weeks (used the app for 4 weeks). Surveys were completed during a virtual check-in via Zoom with a member of the study staff. There was 100% retention of both parents and adolescents throughout the intervention for follow-up surveys.
Adolescents reported on pain interference, peer relationships, physical stress experiences, psychological stress experiences, pain behavior, pain quality, experiences with providers, family relationships, communication, and personal experiences with the SCD. Parents were assessed on disease knowledge, family relationships, family communication, and their experiences managing their child’s SCD. The adolescents were 92% African American, 8% Hispanic, 54% male, and the mean age was 14.8. SCD was not a new disease for any of the participants. The parents were 93% African American, 4% Hispanic, 92% female, and the mean age was 44.9. The small sample size prohibits the investigators from evaluating changes between groups, though summary statistics at each time point were created and reviewed.
In the final surveys, adolescents also reported on the usability of the web app:
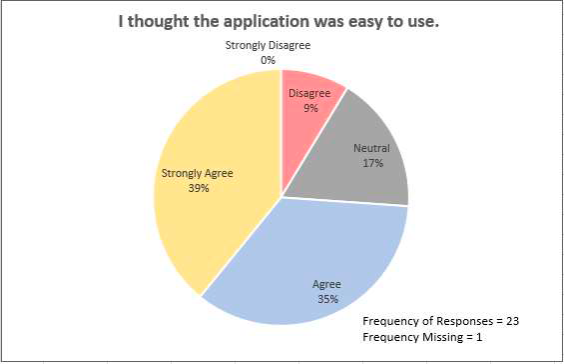
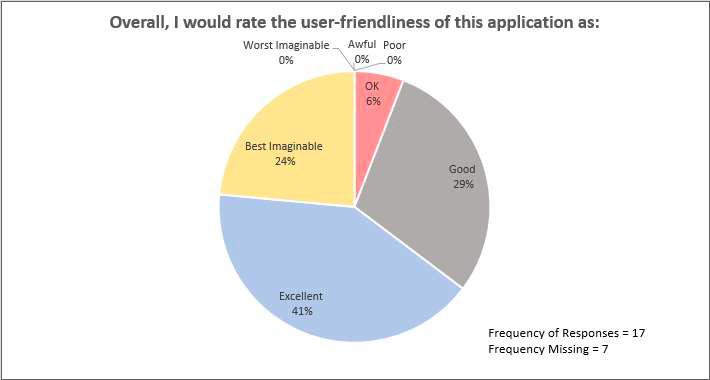
At the baseline and the final survey time points, adolescents reported on communication and SCD pain management:
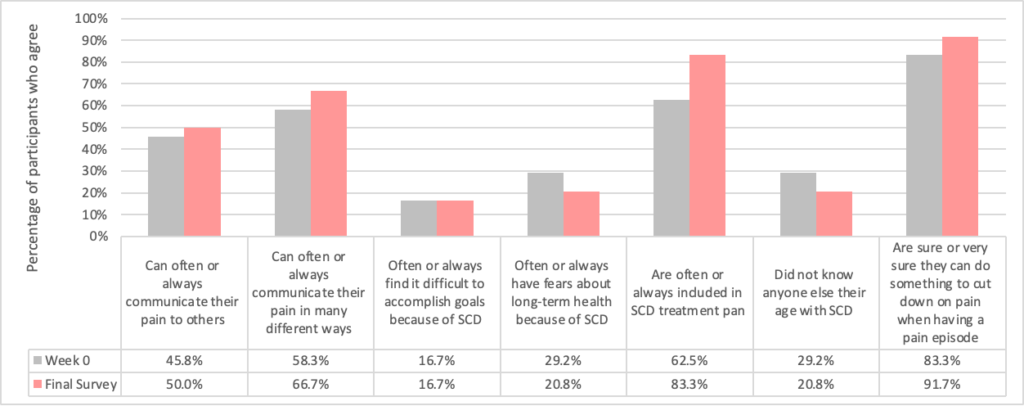
Lastly, web app usage data was collected. From the 24 adolescents randomized, 34 pain reports were entered by 16 unique users (67%) within the app. Of those pain reports recorded, 20 (59%) were shared with their parent or health care provider via the app.
Pinpoint is available to individuals and medical providers. To learn more about the Pinpoint app, check out the video tutorial or visit HPC International.



